| |
| author |
Cristabella Fortna
| | title |
Kinetics and Mechanism of Carbonyl Substitution of Os3(CO)10(μ-I)2 by P(OR)3
| | abstract |
The study of catalysis by organometallic compounds is a well-established field that has more
recently focused on transition metal carbonyl clusters. While transition metal carbonyl clusters are
not inherently catalytically active, they serve as unique structural templates that can be helpful in
designing new homo- and heterogenous catalysts. The introduction of halogens onto carbonyl
clusters has been shown to activate the cluster towards carbonyl substitution. This study reports
the kinetics and mechanism of carbonyl substitution of the dihalo-bridged cluster Os3(CO)12I2 with
P(OR)3 (R = Me, Ph) as a novel structural motif that may be employed or integrated in future
homogenous organometallic catalysts. Substitution of the carbonyls trans to the metal-metal bond
was found to be first order in cluster and zero order in ligand. This substitution is proposed to
proceed through two rate-controlling steps, forming a monosubstituted intermediate compound
Os3(CO)9I2[P(OR)3] before then producing the final compound Os3(CO)9I2[P(OR)3]2. These steps
are proposed to involve the breaking of a metal-metal bond. This reaction was investigated at
temperatures of 49-69 oC and values of k1 were found to range from 1.67 x 10-4 to 2.59 x 10-3 sec-1, and k2 was found to range from 9.31 x 10-5 to 3.47 x 10-3 sec-1 with temperature. Propagated
error of k2 is quite large and thus conclusive statements regarding step two cannot be made at this
time. Each step was found to be independent of ligand identity, and that the rate of the second step
is not affected by the first addition of P(OR)3. The activation energy was found to approximate
120 kJ/mol for the first substitution of each P(OR)3. The propagated error of k2 prevents conclusive
kinetic or thermodynamic analysis of the second substitution of P(OR)3. This investigation
provides a kinetic and mechanistic understanding of the behavior of dihalo-bridge transition metal
clusters that may prove beneficial as a structural template for the design of new compounds and
homogenous catalysts.
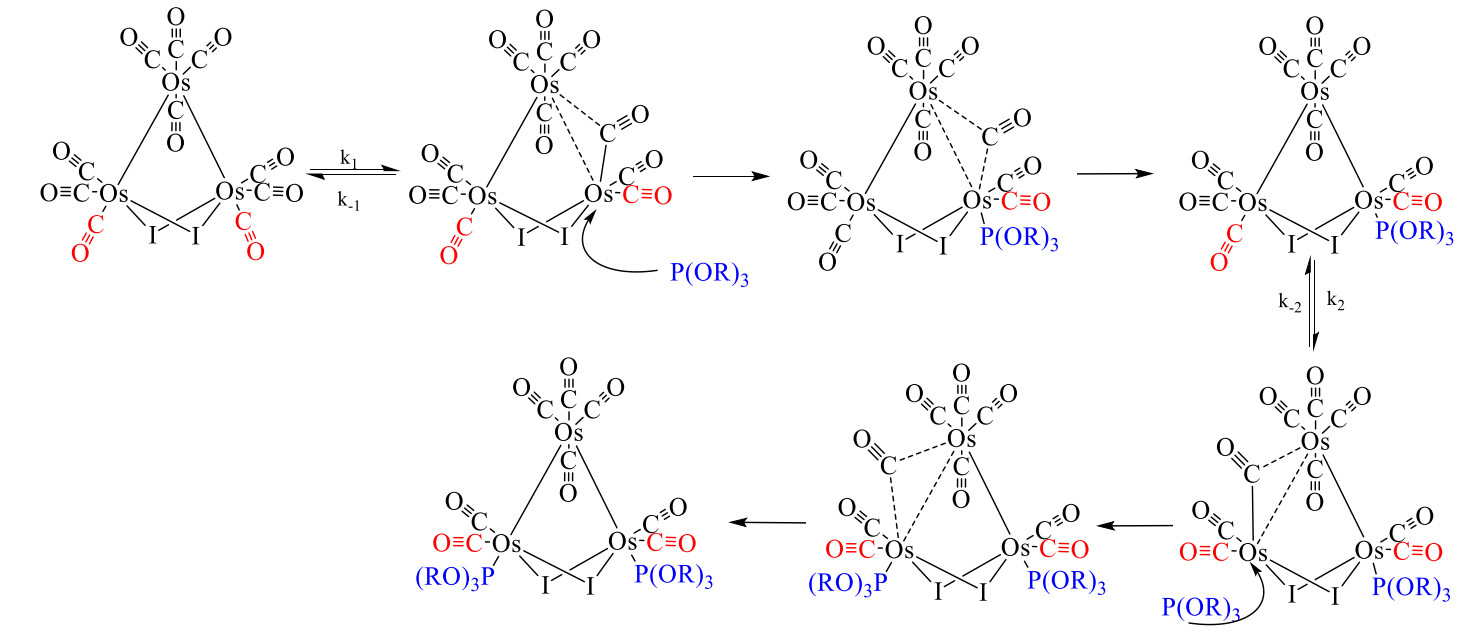
Fig iii. Proposed mechanism of carbonyl substitution by P(OR)3 ligands, consistent with data
reported in this study. The rate-limiting step is proposed to be the breaking of the Os-Os bond, which
is then partially stabilized by an adjacent CO.
| | school |
The College of Liberal Arts, Drew University
| | degree |
B.A. (2023)
|
| advisor |
Dr. Mary-Ann Pearsall
|
| committee |
Dr. Ryan Hinrichs
Dr. Sandra Jamieson
|
| full text | CFortna_Chemistry.pdf |
| |

