| |
| author | Dani Leviss |
| title | The Ozonolysis of α-Terpineol in an Aqueous Environment: A Model for Atmospheric Cloud Chemistry |
| abstract |
Ozone is a major atmospheric pollutant, a central component of smog, a lung irritant, and able to react with abundant organic atmospheric aerosols. The gas phase
ozonolysis of volatile organic compounds has been extensively studied and shown to be a major pathway for the formation of secondary organic aerosol (SOA). Although
recent work indicates that aqueous processes account for a major fraction of SOA, little is known about aqueous phase ozonolysis. In the present research, we studied the
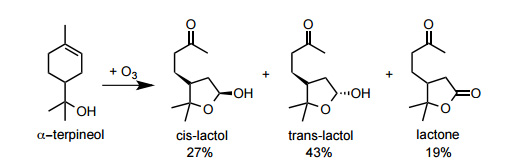
ozonolysis of α-terpineol in aqueous solutions to model the chemistry of atmospheric droplets at varied ozone concentrations (131, 480, and 965 ppb). 1H Nuclear
Magnetic Resonance Spectroscopy (NMR) monitored the experimental progress of this reaction, and one- and two-dimensional NMR along with Gas Chromatography-Mass
Spectrometry (GCMS) and Infrared Spectroscopy (IR) identified products.
The second-order rate coefficient of the aqueous reaction is 9.93 x 106 M-1s-1 with a lifetime
of 5.2 min, 15 times shorter than in the gas phase (lifetime of 79 min). Formation of products of decreased volatility suggests ozonolysis of α -terpineol yields
more condensible secondary organic material and therefore potentially increased impact on climate, visibility, and health.
|
| school | The College of Liberal Arts, Drew University |
| degree | B.A. (2016) |
| advisor | Dr. Ryan Z. Hinrichs |
| committee | Dr. Alan Rosan
Dr. Summer Harrison
Dr. Hilary Kalagher |
| full text | DLeviss.pdf |
| |

