| |
| author | Madeline R Lederer |
| title | A Mechanistic and Kinetic Comparison of the Reactivity of Volatile Organic Compounds on Mineral Dusts |
| abstract |
Laboratory models of atmospheric systems have attempted to account for causes and effects of major dust events in recent decades, but these models lack many key
components of actual dust storms. Elemental analysis of atmospheric dust places silicon and aluminum as the most abundant elements in many storms, leading many to
assume that the oxides of these elements - SiO2 and Al2O3 - accurately reflect mineral aerosol surfaces. More detailed field
studies indicate that aluminosilicate clays are actually the prevalent surfaces in the atmosphere, though these clays are not widely studied in laboratory models.
This work aims to assess the current assumption that Al2O3 and SiO2 reactivity can be used to model aluminosilicate clay aerosols
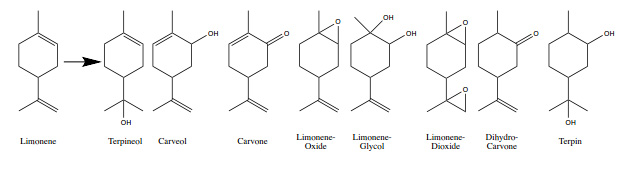
by systematically collecting data on kinetics, product formation, and particle size. Limonene was used as a model volatile organic compound and found to produce a
variety of secondary organic products, as displayed in the figure below, that will be discussed mechanistically with their relation to the reactive Brønsted acid,
Lewis acid, and Redox sites on each dust sample. Our results indicate that clay reactivity differs greatly from that of Al2O3 due to significant
differences in surface structure. Nitric acid was also shown to increase reactive uptake of limonene on mineral surfaces by factors of 6.21 to 16.4, with the greatest
value of γ in the reaction of nitric acid coated kaolinite: 3.656x10-8.
|
| school | The College of Liberal Arts, Drew University |
| degree | B.A. (2016) |
| advisor | Ryan Hinrichs |
| committee | Bai Di
Bjorg Larson
Maryann Pearsall |
| full text | MRLederer.pdf |
| |

